Over 3,000 students and employees have tested positive for the coronavirus since Aug. 1, but according to Melissa Nolan, it's still unlikely the Columbia campus could achieve herd immunity without a vaccine.
Nolan, a clinical epidemiologist and assistant professor of epidemiology and biostatistics in the Arnold School of Public Health, said that for every infectious disease pathogen, there is a certain level of the population that already has protection or antibodies.
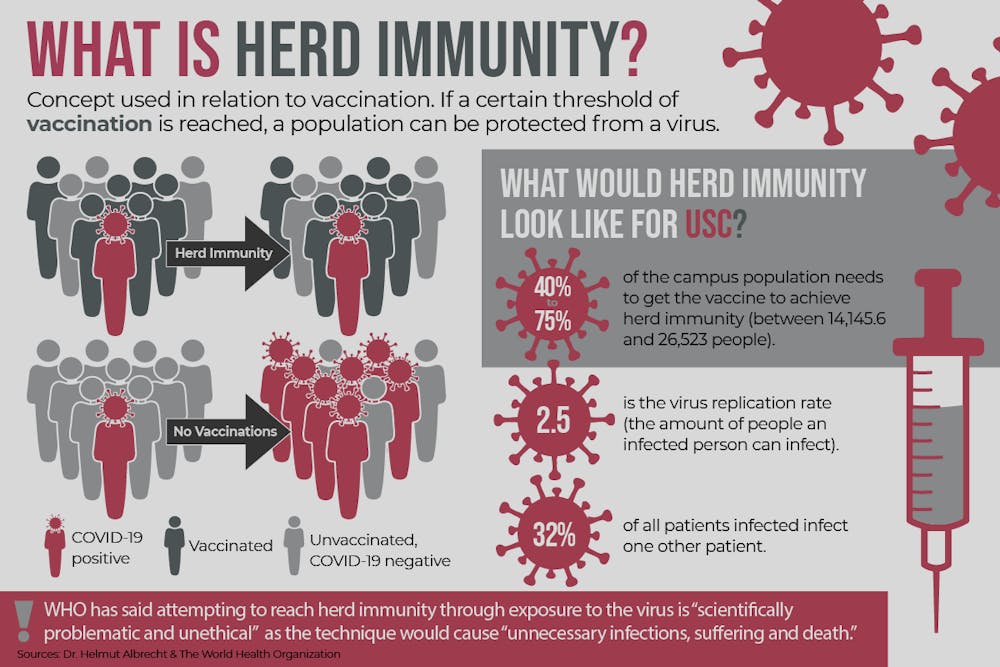
The level of immunity needed to achieve herd immunity among a population varies depending on the disease. For example, with smallpox, 80% of the population would need to be vaccinated for herd immunity to be achieved.
Without a vaccine for COVID-19, herd immunity could only be achieved through transmission of the disease. It's unclear what percentage of a population would have to contract the virus for herd immunity to be achieved, but Nolan said the number could fall "between 40 to 75%."
Lee Pearson, the associate dean for operations and accreditation in the Arnold School of Public Health, said to intentionally strive for herd immunity without a vaccine is a poor public health strategy.
"That would certainly be an unethical approach from a public health standpoint for numerous reasons," Pearson said. "We want to do everything we can to limit disease and reduce illness and prevent death."
Dr. Helmut Albrecht, an infectious disease physician, said for a college population to strive for herd immunity without a vaccine would be very dangerous because the disease would spread beyond the college community.
"These young kids — which will most likely survive this — will come back and go out on the weekends and will go on the main street and go to cafes and other places. They will go home to their grandparents or they will volunteer in a nursing home and infect everybody there," Albrecht said. "So, herd immunity without other intervention is not a plan. That's a plan for disaster."
Dr. Albrecht also said it was important for people to continue to wear masks.
"Until we have a Plan B, which would be a vaccine or would be testing and tracing that would actually capture most people, I think masks are an easy way out," Albrecht said. "Nobody wants to wear a mask, nobody likes masks, but it's safer than dying."
If someone with the coronavirus wears a face covering, it reduces their ability to infect others. However, face coverings are not effective at protecting the user from being infected by others who do not wear a face covering.
"If the person that wears the mask has the disease it is probably over 80% effective. When both of you wear the mask, you're reaching protection around 95%," Albrecht said.
Moderna, Pfizer, its partner BioNTech announced last week they have over 90% effective vaccines, with Moderna even saying no one in its trials of the vaccine developed "severe COVID-19." Typically, a vaccine candidate goes through several clinical trials that test across demographics to determine effectiveness. The process can take years, but with the current circumstances, the timeline has been accelerated.
"The fact that we have multiple vaccine candidates that have been developed and tested through an expedited process, to reveal a successful product within a year's time is nothing short of a miracle really," Pearson said.
The final step for the vaccine candidates is receiving approval from the FDA before the drug is considered safe for the general public. As of Nov. 20, Pfizer and its partner BioNTech have applied to the FDA for an Emergency Use Authorization (EUA) for their COVID-19 vaccine.
The federal vaccine advisory committee will meet on Dec. 10 and Dec. 17 to review Pfizer and Moderna's vaccine data, respectively, before deciding on whether to grant an EUA. An EUA can be granted within 24 to 72 hours after the meetings.